Cryolab is a leading Italian company in the scene of Biobanking and Cryobiology
The group’s figures
Cryolab sites
Cryolab operates at 3 hubs situated in Rome, Pavia and Vicenza, all equipped with highly innovative facilities and staff qualified in the operational management of processes for the storage, traceability and transport of biological samples, monitored 24 hours a day, all year round, by fully automated systems.
Rome
Via Montpellier, 1
PAVIA
Viale Certosa, 10
COSTABISSARA (VI)
Via Antonio Meucci, 26
Certifications
ISO 9001: 2015
Provision of services for the hospital sector: organisation and management of the transportation of biological samples, management of biobanks and cell manipulation laboratories, biological sample preservation, disaster recovery plan and disaster recovery services
ISO 21973: 2020
Provision of the service for the transport of cells for therapeutic use and research
Health Ministry approval
Health Ministry approval for the storage and transport of biological samples, and management of the Disaster Recovery Plan
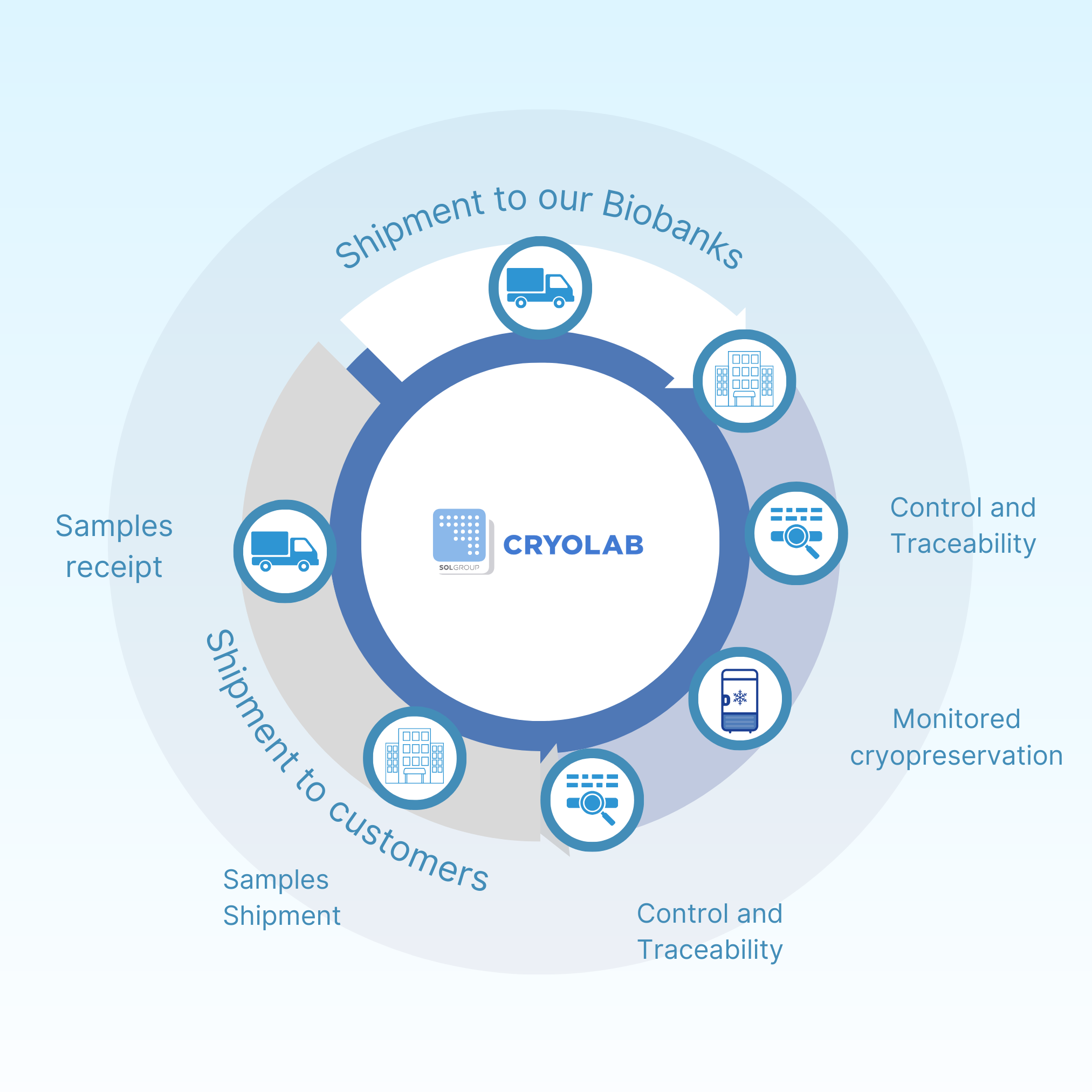
Services
Our team
The Cryolab team is made up of highly qualified professionals who ensure safety and efficiency in the transport and preservation of biological samples. The cross-sector training and experience of each team member, together with the support of SOL Group colleagues, guarantees the management of complex situations and a high quality service for our customers.

Alba Gorenca
Site ManagerWith a degree in Chemistry and Pharmaceutical Technology, she has collaborated with leading companies in the pharmaceutical industry, covering the roles of Technical Manager, Quality Assurance Officer and Lead Auditor. At Cryolab since 2021, she is now Site Manager.

Francesca Agostini
Quality Assurance Officer and Cryobiology Room DirectorWith a degree in Biology and specialised in Clinical Biochemistry, she collaborated for years with the Tor Vergata University General Hospital, Rome, at the Cell Therapy Laboratory, where she obtained certification as a Qualified Person. At Cryolab since 2017, she is a Quality Assurance Officer and Cryobiology Room Director.

Nicola Daniele
Logistics Service ManagerWith a degree in Cellular and Molecular Biology and specialised in Clinical Pathology, he collaborated for years with the Department of Oncology and Haematology of the Bambino Gesù Children’s Hospital in Rome. With Cryolab since 2012, he covers the role of Logistics Service Manager, and is professionally certified for the international transport of goods in accordance with IATA directives.

Simona Ercolani
Sales AdministratorWith a degree in Communication Sciences, she has extensive experience in multinational companies operating in the field of Sales Administration, in which she continues to operate at Cryolab.

Luca Novello
Plant and Instrument ManagerWith a degree in Biomedical Engineering, he has collaborated with the Tor Vergata General Hospital of Rome, in the management of biomedical devices. At Cryolab since 2020, he covers the role of Plant and Instrument Manager.

Angelo Petrivelli
Logistics OperatorWith a background in tourism and cultural studies, he has covered the role of Logistics Operator at Cryolab since 2019.

Claudio Spinucci
Logistics OperatorHe has covered the role of Logistics Operator at Cryolab since 2021.

Giuseppe d’Agnese
Logistics OperatorHe has covered the role of Logistics Operator at Cryolab since 2021

Sara Vassallo
Sales ManagerContacts
Work with Cryolab
Working with Cryolab will enable you to embark on a professional career within the SOL Group, in a dynamic multinational company that is steadily growing in the international scene.
We are looking for talented people capable of enriching Cryolab by giving them the opportunity to grow professionally in a dynamic and collaborative environment.
FAQ
Competence
Cryolab has decades of experience and competence in the field of cryobiology and preservation of biological samples. Familiar with the complex legislation regulating the preservation of samples, the company ensures that samples are preserved in accordance with the applicable protocols.
Cost savings
By outsourcing the preservation of samples, there is no need to purchase and maintain equipment and plants, or to procure liquid nitrogen.
Safety
Outsourcing ensures that the samples are preserved in a safe environment, guaranteeing the necessary degree of safety.
Flexibility
Outsourcing storage enables organisations to gain access to any sample whenever it needs to from anywhere in the world.
Reliability
Cryolab ensures that samples in its possession are preserved under the best possible conditions, so as to guarantee their long-term integrity and stability.